CPHI Worldwide in Barcelona
Our company will exhibit at the CPHI Worldwide in Barcelona in October 24-26. This event is the world's most important gatherings of the pharmaceutical industry. It is a great opportunity to meet with new clients as well as discuss current and future business...
An Investigation of Atropisomerism of Amenamevir and Its Intermediates by Dynamic HPLC
An Investigation of Atropisomerism of Amenamevir and Its Intermediates by Dynamic HPLC.
Patriapharma will be at Chemoutsourcing
Our company will be represented at the Chemoutsourcing event in USA, Parsippany, NJ Hilton. The event will take place until September 8. Visit us at booth 9.
Exhibitors on the CPHI
CF Pharma and Patriapharma will be exhibitors on the CPHI Japan event.
Exhitibitors at CPHI Japan
Our company member Alex Gruman and Patriapharma-Japan office manager Shigeru Oshikiri are present as exihibitors on the CPHI Japan from April 19-21
Start of a new year
We have started the new year with welcoming Mr. Alex Gruman into the Business development director role. Alex will share his 35+ years experience in pharma business where he served different CDMOs as managing director as well as his long period within Rhone Poulenc...
Atracurium
Due to the Covid-pandemic a large volume of Atracurium was needed worldwide. Government agencies and private companies ordered huge amounts from the API. Atracurium is a muscle-relaxant mostly used for respiratory infections during the anesthesia procedure. It was a...
GMP Certificate for CF Pharma Ltd.
Successful renewal of the GMP Certificate for CF Pharma Ltd. Three members from the National Institute of Pharmacy (Hungarian: Országos Gyógyszerészeti Intézet, known as: „OGYI”) audited our companies for GMP compliance on 5 APIs (Atracurium besylate, Cabergoline,...
CPHI Worldwide in Frankfurt
Our company was represented on the CPHI Worldwide in Frankfurt. This was the first major pharmaceutical global event after the Covid pandemic, which was held live. It was a great pleasure to meet with new clients and discuss current and future business endeavours.
Hungarian Business Leaders Forum
Company CEO, Zita Schneider became a proud member of the Hungarian Business Leaders Forum (HBLF), and she attended their event as a member of Patriapharma Ltd.
R&D director
Imre Kovács now became the R&D director of our company. He will be leading the R&D-team, where he will oversee new synthesis, new technologies and CDMO production. CF Pharma now possesses 3 laboratories.
Site Accreditation Certificate
CF Pharma has successfully renewed the Site Accreditation Certificate, with this document out company is allowed to sell APIs to Japan territories.
Japanese GMP
The Japanese GMP Compliance Inspection Result Notification is successfully received.
Establishment of Patriapharma Japan
We are proud to announce that our commercial partner Patriapharma established its Japanese subsidiary. Patriapharma Japan Co., Ltd. which was founded on 28/12/2020 and was created with the express purpose of meeting the strict Japanese regulations and providing...
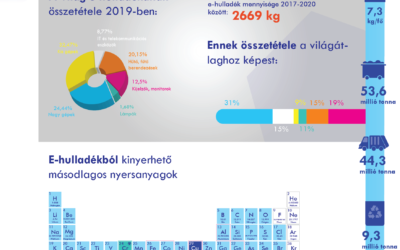
Electronic waste recycling method
We pays special attention to waste management, whether hazardous waste from production or other waste indirectly related to production. Accordingly, an integral part of our waste management is the selective collection of electronic waste and its transfer to a...